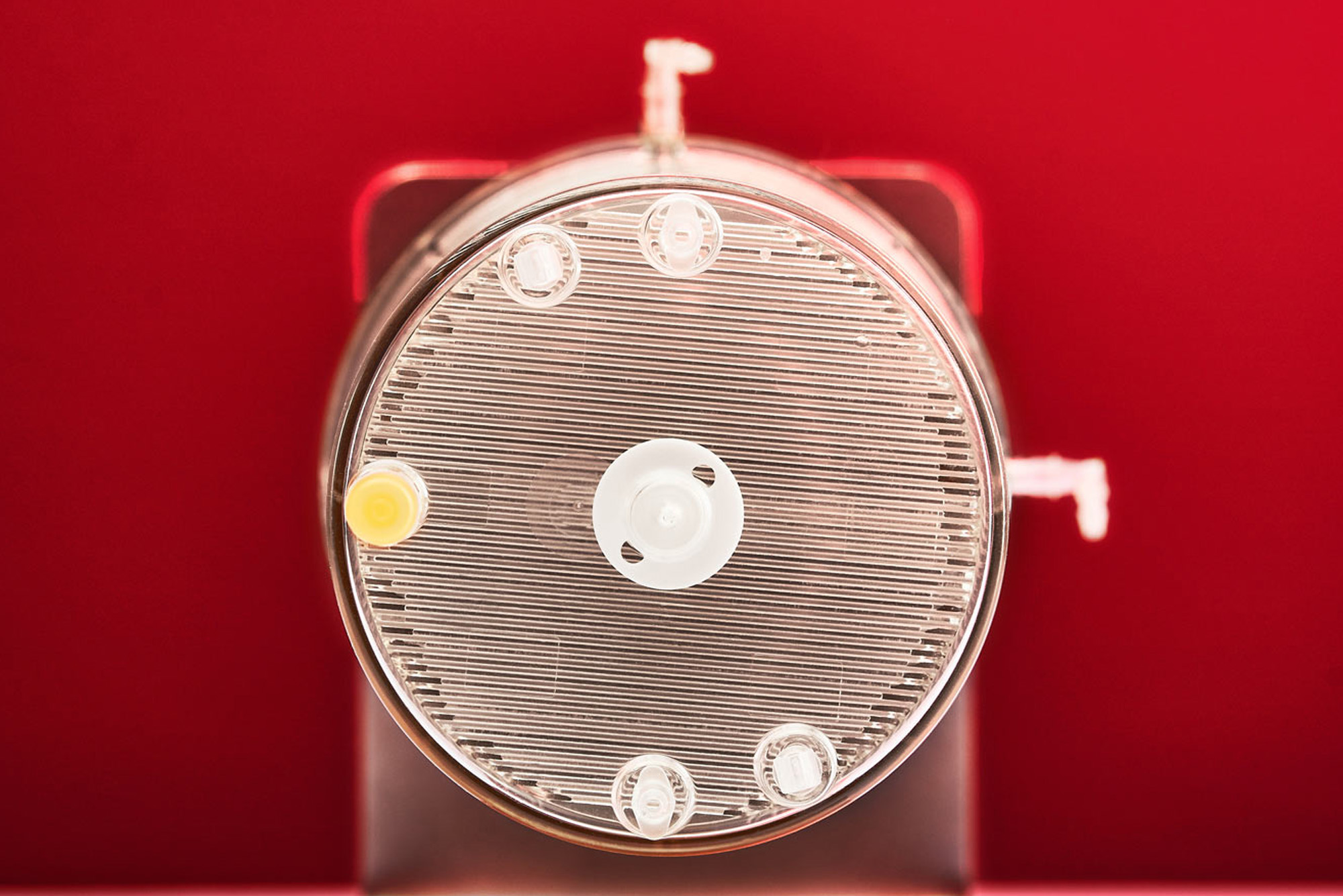
ZRP Cultivation System
Cultivation of human cells in high yields and proved functionality
Zellwerk´s ZRP platform and the belonging perfusion bioreactors have proven as a potent and reliable instrumentation for ex vivo expansion of all human blood and tissue derived cells we tested. For manufacturing needed amounts of nonadherent or adherent growing cells suited single use perfusion bioreactor types are available. Cultivation in perfusion modus turned out profitably related to yields, functionality, consumption of medium, compared to cultivation under static conditions or in turbulent medium flow. Cells listed here can be produced in wanted amounts and qualities by customer order.

Main advantages
Main Advantages of Zellwerk’s ZRP Cultivation System
Mass amounts of immune cells and stem cells are regularly produced in a totally closed perfusion bioreactor system (multiple 10^9 to multiple 10^10 cells).

GMP processes are worked out for patient-individual T cells and TIL, for haploidentical NK cells, for mesenchymal stem cells/stromal cells from many sources.

The immune cells- and stem cells cultured in our perfusion bioreactors with their directed, laminar and controlled medium flow have much longer ability for doubling and growth compared to the same cells growing under static conditions or turbulent medium flow.

The ZRP bioreactor series consist of totally closed bioreactor types. Once cells for ex vivo expansion are seeded, they grow under safe and sterile conditions until they are harvested into a connected bag and confectioned as an ATMP. Zellwerk has manufacturing license for proving T cells, NK cells and MSCs in clinical trials, the manufacturing license for TIL is applied for.
ZRP Bioreactors
Propriety features of the ZRP Cell Cultivation Platform and Technology

Any ex vivo expansion of immune cells, stem cells, induced pluripotent stem cells, functional organ-specific cells for therapeutic use requires certain specific conditions to produce them in needed quantities as well as with wanted functionalities. Culture processes should be utmost standardized and reproducible, identical phenotypes and composition of sub-populations should be aspired (so far inherent diversity does not hinder that).
The ZRP cell cultivation system stands out by several innovative, mainly technical features allowing to explore optimized culturing conditions for each cell type and guarantees reproduction for any once adapted cultivation process. Zellwerk has built up a comfortable working place (low footprint) suited for patient-near manufacturing mass amounts of all common immune cells and stem cells (even as personal ATMP). The platform consists of a GMP Breeder (which is also a laminar flow bench) providing power, medium, gasses, sensors; a Control Unit with touch screen (to choose, steer and control a cultivation process); a proprietary software (documenting and evaluating all process data).
Zellwerk´s series of single use perfusion bioreactors can be operated on the platform under GMP conditions (for example in a class D clean room). Continuous automated control of pH, pO2, temperature in the bioreactor medium is assured; fresh medium feeding, rate of laminar medium circulation to fresh medium feeding, garbage outflux are automatically regulated by algorithms.
The ZRP cell cultivation system stands out by several innovative, mainly technical features allowing to explore optimized culturing conditions for each cell type and guarantees reproduction for any once adapted cultivation process. Zellwerk has built up a comfortable working place (low footprint) suited for patient-near manufacturing mass amounts of all common immune cells and stem cells (even as personal ATMP). The platform consists of a GMP Breeder (which is also a laminar flow bench) providing power, medium, gasses, sensors; a Control Unit with touch screen (to choose, steer and control a cultivation process); a proprietary software (documenting and evaluating all process data).
Zellwerk´s series of single use perfusion bioreactors can be operated on the platform under GMP conditions (for example in a class D clean room). Continuous automated control of pH, pO2, temperature in the bioreactor medium is assured; fresh medium feeding, rate of laminar medium circulation to fresh medium feeding, garbage outflux are automatically regulated by algorithms.
ZRP Bioreactors
Zellwerk’s Proprietary Bioreactors
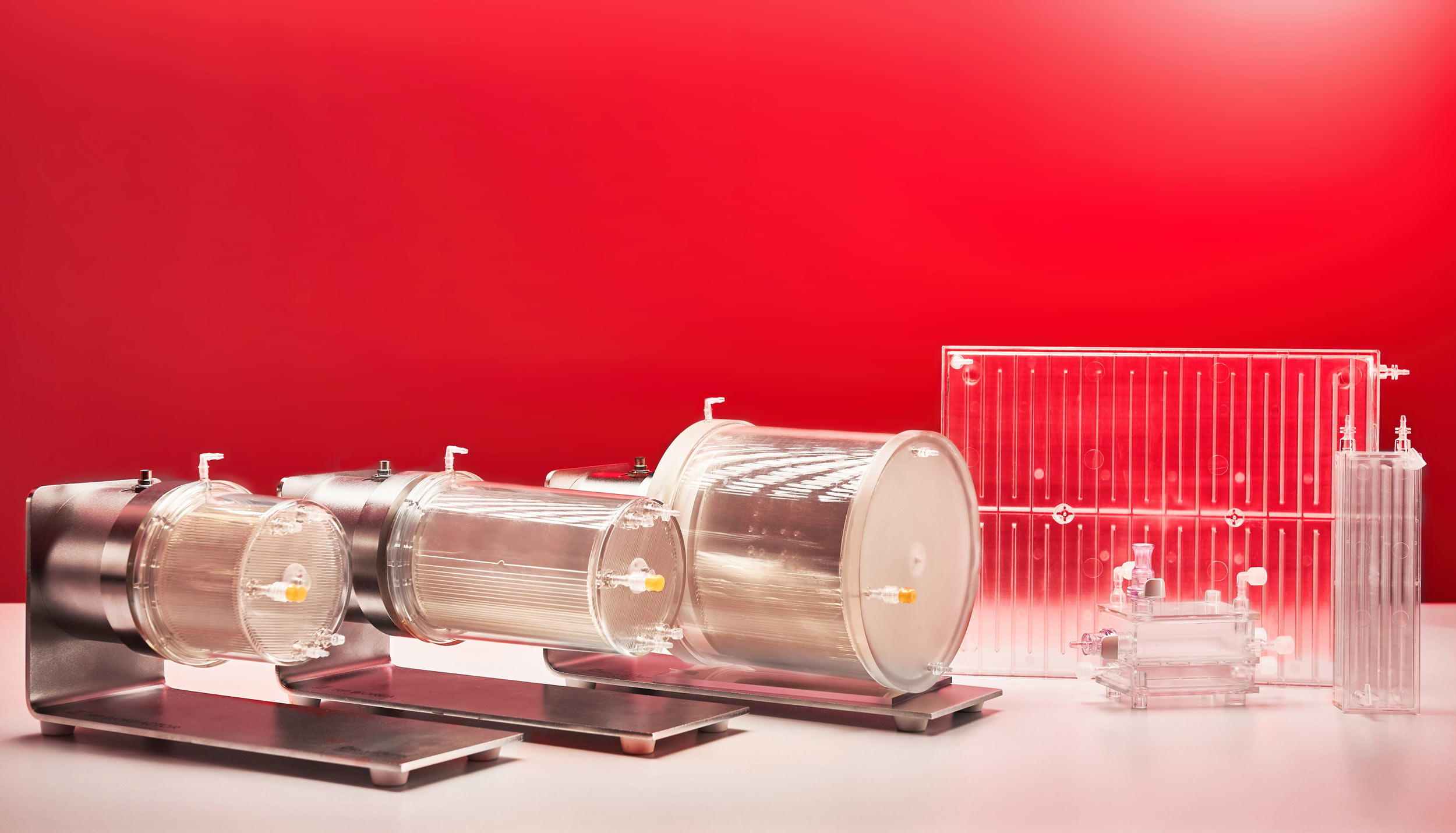
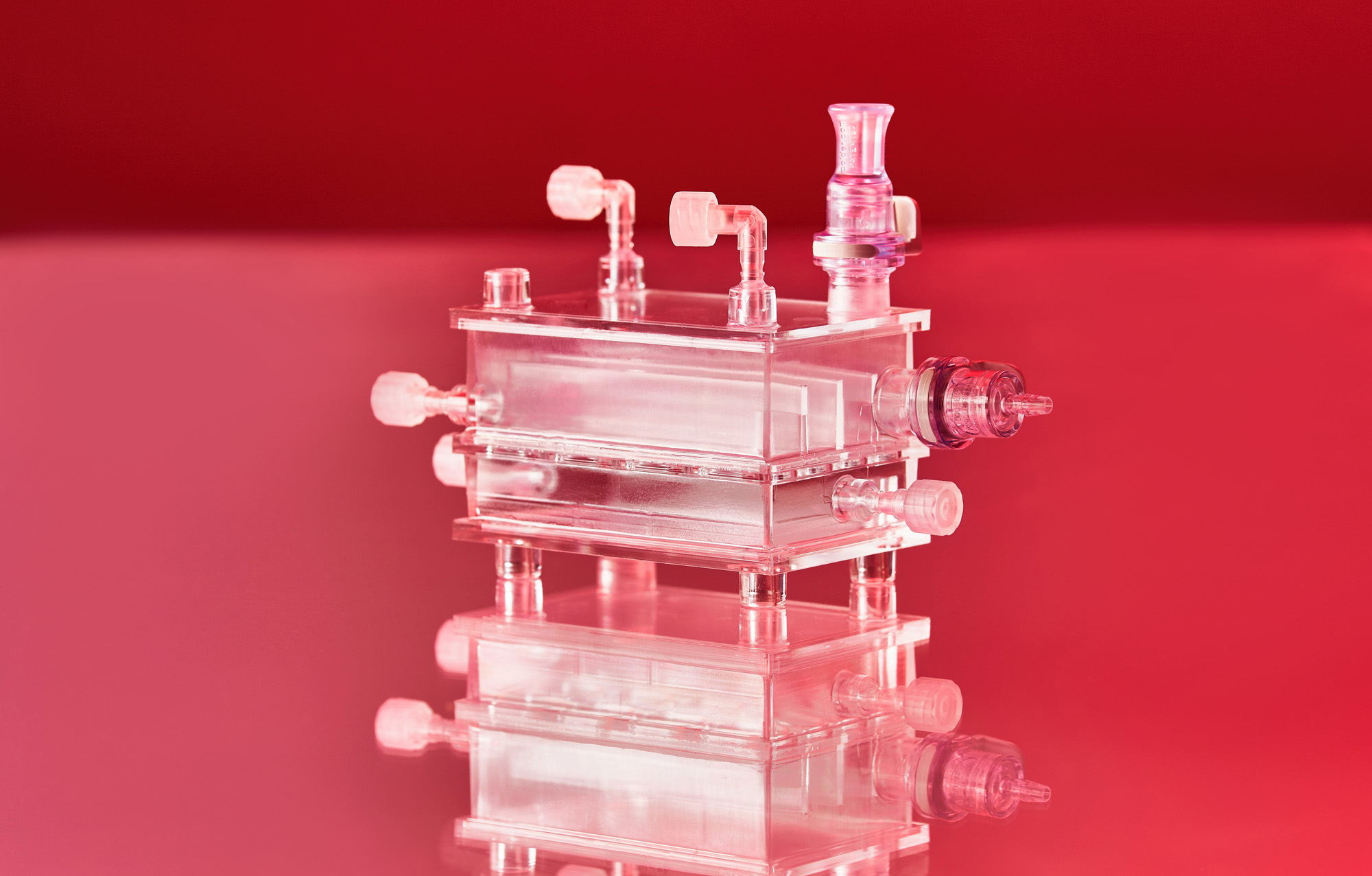
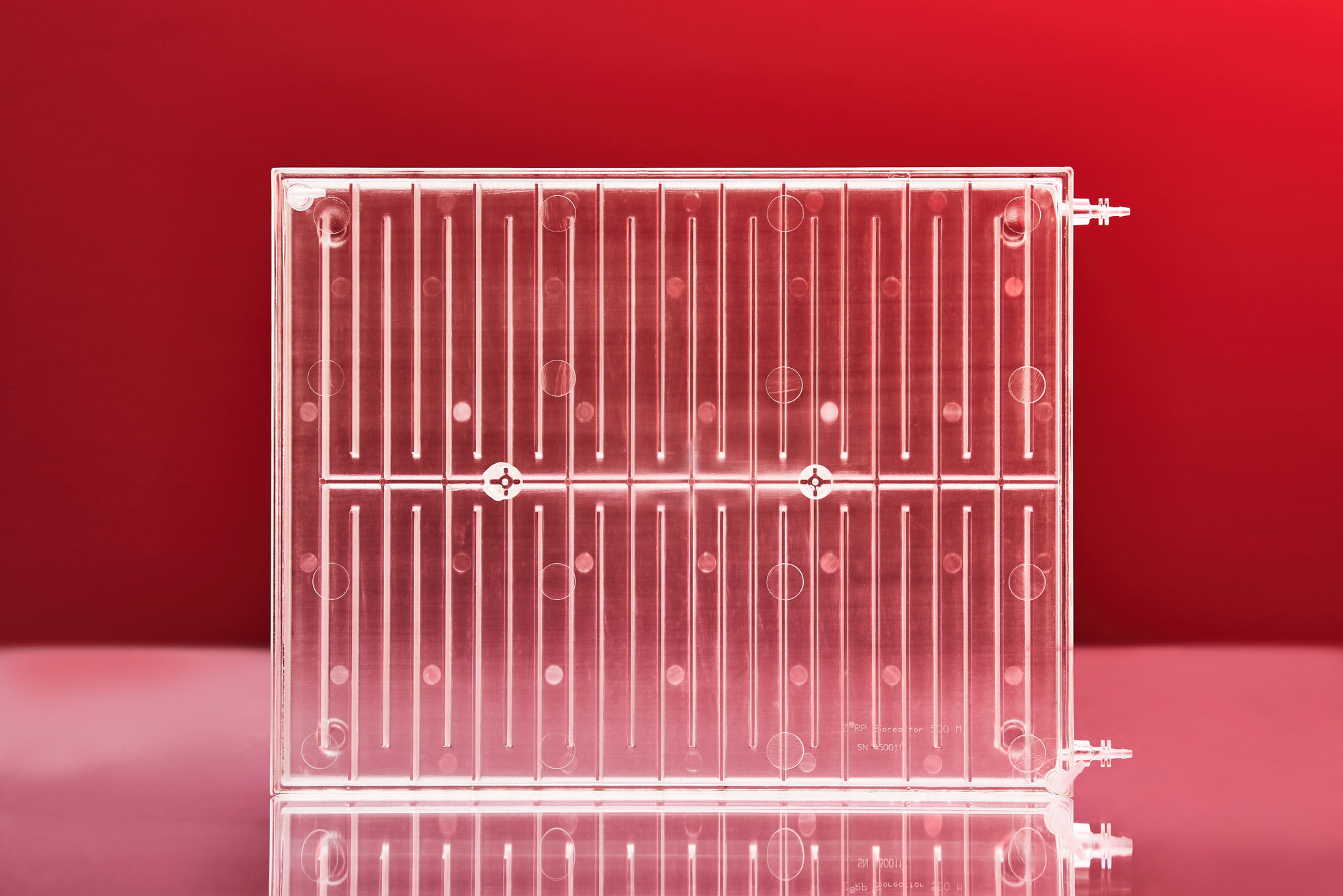
Zellwerk has developed several innovative types of perfusion bioreactors (all patented). We fabricate an innovative perfusion bioreactor series having different volume and equipment for normoxic, hyperoxic or hypoxic culturing. The single use ZRP meander type perfusion bioreactors show exiting properties to expand non adherent growing human cells in amounts commonly used for treatment of patients in clinical studies (see table). Cultivation processes for T cells, TILs, NK cells have proven very reliably and reproducible in many hundreds of cell expansion runs. A second series of single use bioreactors possess a horizontal cell carrier bed with extremely high surface in a low volume vessel for expansion of adherent growing cells. After seeding and adhesion of the cells the cell carrier bed is rotating in the medium containing culture vessel.
The immune- and stem cells cultured in our perfusion bioreactors with their directed, laminar and controlled medium flow have much longer ability for doubling and growth compared to the same cells growing under static conditions or turbulent medium flow.
The immune- and stem cells cultured in our perfusion bioreactors with their directed, laminar and controlled medium flow have much longer ability for doubling and growth compared to the same cells growing under static conditions or turbulent medium flow.
ZRP Bioreactors
Zellwerk’s Meander Type Bioreactor Series
The meander type bioreactors mimic the flow of blood in blood vessels. Medium flow is directed and laminar and does not stress containing cells. Seeded cells or tissue pieces sediment on the ground of the meander bed, getting in touch with each other and with the surface of the stream bed. Concentration of nutrients remains homogenic in the medium volume over a cultivation run by algorithm steered rate of circulating medium to fresh medium feeding. Sedimented cells move slowly in the total medium stream comparable to trafficking of cells in natural blood vessels.
Optimized activation phases Accelerated expansion and is archived By and specific activation Expansion can be of (having a specific coating or not). Fresh medium influx and cell broth outflux is regulated dependent on the upgrown cell number. A steady state of nutrients and gasses (oxygen) in the total medium volume in a bioreactor run is achieved by slow internal medium circulation. Five meander type bioreactors are available. Cultivation protocols for specific activation, accelerated expansion, gaining preferred immune cell phenotypes have been worked out.
Optimized activation phases Accelerated expansion and is archived By and specific activation Expansion can be of (having a specific coating or not). Fresh medium influx and cell broth outflux is regulated dependent on the upgrown cell number. A steady state of nutrients and gasses (oxygen) in the total medium volume in a bioreactor run is achieved by slow internal medium circulation. Five meander type bioreactors are available. Cultivation protocols for specific activation, accelerated expansion, gaining preferred immune cell phenotypes have been worked out.
ZRP Bioreactors
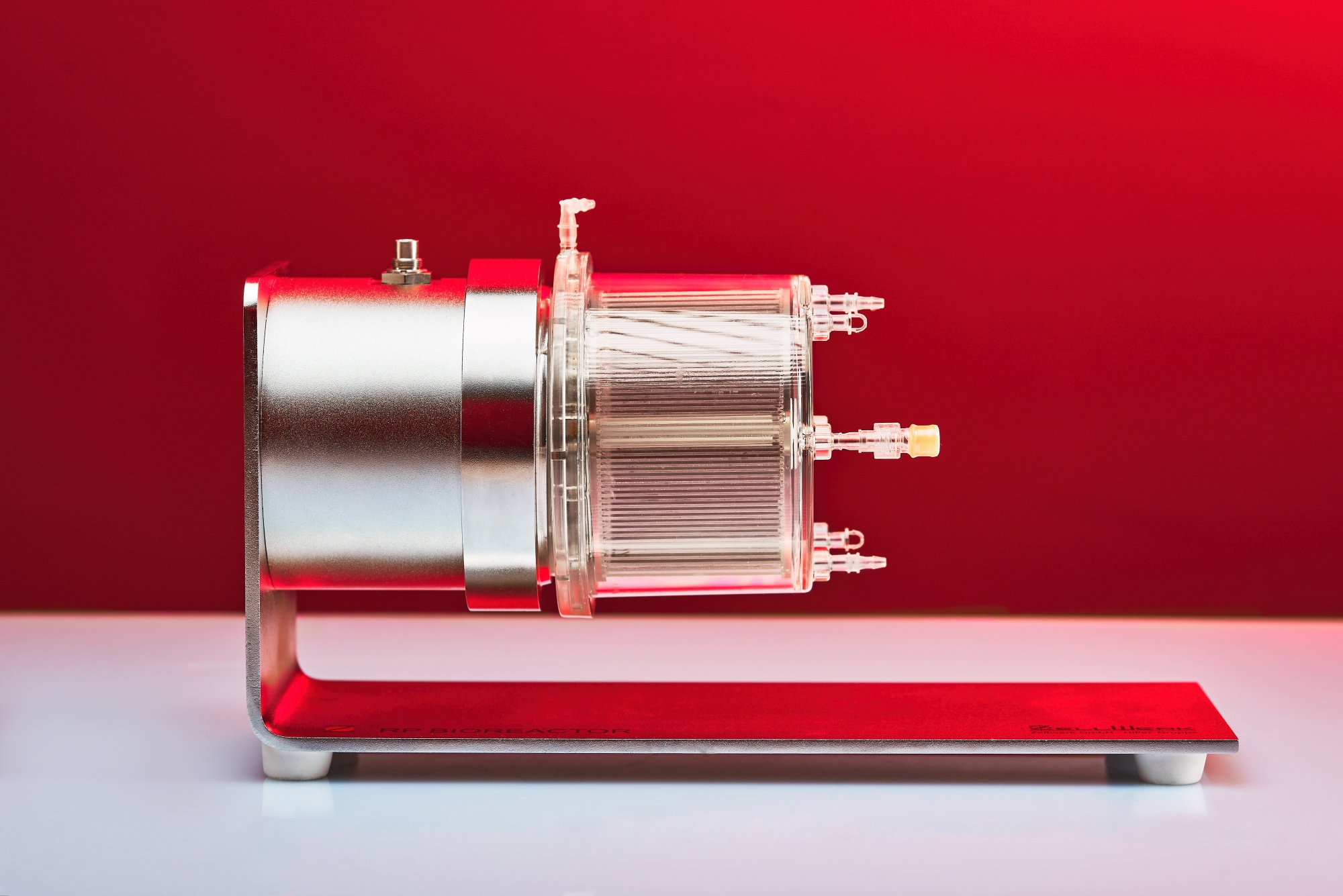
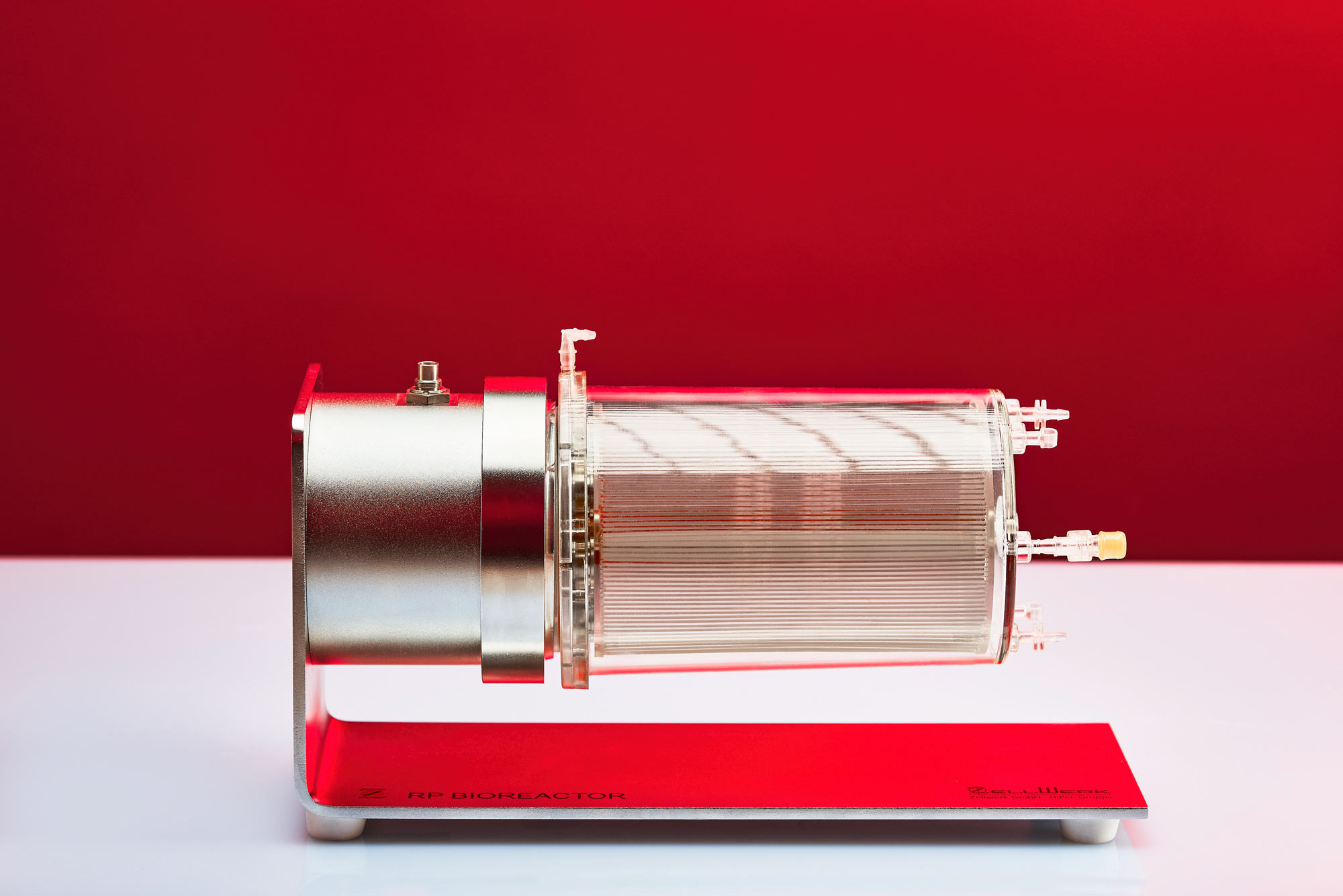
Zellwerk’s Horizontal Bed Perfusion Bioreactor Series
The totally closed H type perfusion bioreactors enable in vitro expansion of adherent growing stem cells/stromal cells/endothelial cells from different origin. In arrested horizontal position these perfusion bioreactors provide a huge surface to seed adhering cells on both sides of the cell carrier sheets. Once adhered slow circulation of the bed leads to laminar medium flow with directed cell growth and no cell stress. Homogenous provision with nutrients as well as gassing is automatically steered by an algorithm.
Often used MSCs from all common sources like mesenchymal stem cells from umbilical cord tissue, umbilical blood, uterus tissue, fat tissue, cartilage tissue can be expanded to meaningful therapeutic amounts. In case of bone marrow derived MSCs we succeed regularly to expand the contained MSCs from a little starting aspirate without any passaging step. There is no reporting in the literature on such high numbers of MSCs from human bone marrow expanded to more than 1010 numbers of MSCs (see table). Three single use horizontal bed perfusion bioreactors are available.
Often used MSCs from all common sources like mesenchymal stem cells from umbilical cord tissue, umbilical blood, uterus tissue, fat tissue, cartilage tissue can be expanded to meaningful therapeutic amounts. In case of bone marrow derived MSCs we succeed regularly to expand the contained MSCs from a little starting aspirate without any passaging step. There is no reporting in the literature on such high numbers of MSCs from human bone marrow expanded to more than 1010 numbers of MSCs (see table). Three single use horizontal bed perfusion bioreactors are available.
Continue to Career